Spörndly R, Eriksson S, and Godskesen T. Representation of ethnic minorities in Swedish clinical cancer trials: a qualitative study of physicians’ experiences. Harvard Public Health Review. Fall 2018;20.
DOI:10.54111/0001/T1
International literature shows ethnic minorities and immigrants being underrepresented in clinical trials. This compromises the generalizability of the results and distributes the benefits of participating unequally. This problem is unexplored in Sweden. Therefore, this explorative qualitative study examines the barriers Swedish physicians encounter, the strategies they use to prevent and circumvent the issue, and the attitudes and perceptions they have. We found that physicians do encounter ethnic minority patients that they exclude from participation in clinical cancer trials. This is primarily because of language barriers preventing patients from understanding participant information. Conscious strategies to counter this are lacking. A lack of translated material and strict inclusion criteria are two obstacles that can be overcome. The general conception is that this issue is uncommon and unimportant from a medical perspective, but questions of fairness have been raised. For such reasons, further discussion and research on this issue are needed.
When selecting clinical trial participants, a sample is taken to be representative of the investigated population. However, there is a reason to doubt the representativeness, as there historically has been an underrepresentation of ethnic and linguistic minorities in the US and elsewhere, that remains to this day (Chen et al., 2014; Eshera et al., 2015; Murthy et a., 2004).
There is convincing evidence that clinical and ethical issues can follow from such underrepresentation. First, as noted, if a study sample is not diverse enough, representativeness becomes compromised (Britton et al., 1999; Kurt et al., 2016; Woodward-Kron et al., 2016). Second, studies have shown different pharmacodynamics and pharmacokinetics due to genetic composition variations among patients. There is a difference in the metabolism of therapeutic agents depending on which allelic variants are expressed in an individual, variants that might vary between ethnic groups. Ethnically dependent differences have also been found in outcomes for patients treated with chemotherapy. The differences might have intrinsic or extrinsic causes and call for caution in judging transferability (Chen et al., 2006; Phan et al., 2009). Third, there is an issue of inequality in treatment access. Granted, trials primarily aim to assess effectiveness or side effects, but there are those who do benefit from new experimental treatments. Additionally, patients in clinical trials might wish for or appreciate having a higher frequency of tests, revisits or follow-up phone calls (Ford et al., 2008; Giuliano et al., 2000; Hussain-Gambles et al., 2004). Fourth, some cancer types are more common in certain minority populations (Giuliano et al., 2000), and in failing to include these populations in research on the very disease from which they suffer, researchers compromise the usefulness of the results (Ford et al., 2013).
The literature shows ethnic minorities to be underrepresented in international clinical trials (Chen et al., 2014; Eshera et al., 2015). In Sweden, personal data regulations and laws prohibit the collection of ethnic data (SFS 2008:355), so knowledge on the issue is limited. As the Swedish demographics have changed significantly with an increasing number of foreign immigrants, it is crucial to investigate doctors’ views and experiences of recruiting ethnic minority participants to clinical trials. This study aimed to explore the barriers to, the strategies for, and the ethical implications of the inclusion of people from ethnic minorities (such as immigrant families) in clinical trials. To our knowledge, this study is the first of its kind in Sweden.
This is an explorative, qualitative study. A university hospital in Sweden was selected due to convenience sampling; the hospital was accessible to the researchers within a reasonable distance and administrated a lot of clinical trials. Division heads approved of the study. No ethical review was needed or possible as the project did not fall under the Swedish Act on Ethical Review. It was not invasive, or risky, or collecting any sensitive data as defined in the act (SFS, 2003:460). Written informed consent was obtained in line with the Declaration of Helsinki (World Medical Association [WMA], 2013). Inclusion criteria were doctors working in oncology and hematology clinics with both care and trials. Of twelve doctors consenting to participate, eight doctors subsequently did. Four were female and four male, with significant variation in age. Seven were specialist doctors (Table 1).
The semi-structured interviews took place in 2017. The interview guide was constructed for the study and drew upon previous research in the field, with the aim of covering the main ethical issues of concern to physicians. It was inspired by Hansagi and Allebeck (1998), consisting of four open questions on recruiting ethnic minority participants to clinical trials (Table 2). No technical definition of ‘ethnic minority’ was used, instead, the questions were framed as being about ‘patients with a non-Swedish background’ in order to elicit as broad a response as possible. Interviews lasted for about 60 minutes and were then audiotaped and transcribed verbatim.
Interview transcripts were analyzed inductively with content analysis (Burnard et al., 2008). The first step included multiple careful read-throughs of the content. Phrases summing up relevant content were noted. These were then compiled in broader themes, labeled, and grouped to answer the study questions. The coding resulted in a list of 203 codes and phrases summarising the transcripts. Following analysis, duplicates were crossed out and remaining codes sorted into more extensive categories.
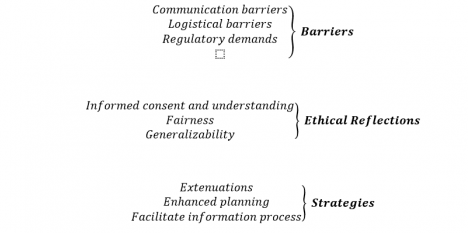
The analysis describes barriers to, strategies for and the ethical implications of the inclusion of ethnic minority patients in clinical trials according to nine categories (Fig. 1).
Communication and language barriers to the inclusion of patients from other ethnic backgrounds were widely recognized. All interviewed mentioned difficulties including patients in studies when not speaking the language: “It’s the language then? It’s the language! And from your side, that means…? We don’t ask them” (Interviewer, Doctor 2).
As one doctor put this; if they do not speak Swedish “then I know they’re not eligible for all types of trials …” (Doctor 6).
Limited Swedish proficiency was perceived as challenging for understanding the details of research participation, such as appointments and referrals. This may lead to study dropout, a reason to select other patients instead:
“It’s the way of least resistance. I know it’s not just difficult to include them; it’s also difficult to do the follow-up. That’s the hardest part. All of a sudden, they don’t show up for the radiology appointment, and they don’t answer their phone … they’ve misunderstood because everything has to go through a third party…” (Doctor 3).
Logistics were mentioned as a barrier, typically the extra work needed to include patients not speaking Swedish: “I think it’s easier to exclude patients of non-Swedish origin because there is so much work included” (Doctor 5). Often there would be many patients eligible for the study, which led to the exclusion of those who would add more to the workload: “But when we’re conducting academic studies there is a lack of funding, and nobody has a shortage of patients in any way so that leaves out those who aren’t cast in the same mould …” (Doctor 3).
Time and resources would have to be spent on using interpreters and translating the written information: “… All papers and documents have to be translated into the proper language and such. I think that can be an obstacle, that it takes too much time” (Doctor 5).
The study design was another major factor reported to negatively influence the participation rate of patients with non-Swedish backgrounds. In many study protocols, not being proficient in Swedish works as an exclusion criterion: “Since we have the written information in Swedish, we have very few [patients] that aren’t Swedish speakers. This is often an exclusion criterion; that you can’t participate if you can’t understand Swedish” (Doctor 2).
Demands on the consent information result in it often being long and complicated, so even those fluent in Swedish can find it difficult to understand: I don’t think those who are badly informed are so because they’ve been fooled, but on the other hand, it might be that we fool them by handing out patient information that is so long and intricate that they haven’t been given a chance to understand” (Doctor 7).
Some interviewees highlighted that the study design did not allow information to be communicated just orally and that any written information must be approved by an ethics committee, and this for every language used. This requirement made it difficult or even impossible for the clinical researchers to translate the material themselves or to use an interpreter.
Most doctors had a positive attitude towards including patients in research. Some noted how patients from ethnic minorities seem more willing to participate in studies due to a high level of trust in physicians. However, some underlined the importance of informed consent and that the patient must fully be able to understand what research participation entails. This moral demand resulted in an ethical challenge when patients’ will to participate or their ability to understand were questioned. The general eagerness to include as many patients as possible on the one hand, and the hesitance to the inclusion of patients where their understanding was questioned on the other, resulted in swaying opinions and uncertainty when confronted with the interview questions: “Everybody should get the same opportunity [to participate] … But I have to be sure they comprehend the information about what it means, regardless of language – or other barriers” (Doctor 5).
The complexities of informed consent sometimes led to frustration among the interviewed doctors, especially when a patient agreed to participate but there were still some doubts about their comprehension: “You don’t know how much they’ve listened and you definitely know they haven’t had time to read everything. What are you supposed to do? Can you command them to read and understand!? (Doctor 8)
Participation in clinical trials was often considered beneficial for patients because of the increased number of visits, tests and the higher level of care, as well as the potential positive effects of new treatments. This led to considerations of fairness, where some found it unfair to exclude patients lacking language skills: “Yes… that’s true, this group doesn’t get access to clinical trials that most people find great” (Doctor 2).
Not all agreed. Another interviewee pointed out that clinical trials are not meant to provide benefits even though many patients might find participation beneficial. This fact makes questions about fairness irrelevant: “In studies, it’s more that they aren’t meant to be done for the sake of the patient primarily; it’s for the sake of science. Patients should participate, but if you’re going to be strict, it’s not for their own benefit. Then it’s equally unfair or discriminating” (Doctor 7).
The interview subjects did not see generalizability as a problem when it came to ethnicity. Some reflected that there always will be a discrepancy between the study sample and the population due to the eligibility criteria that define the patient population under investigation; to allow effectiveness assessments in a well-defined population, those criteria will always exclude some groups because of comorbidities or concomitant treatment, for example. This discrepancy was held to have a more substantial impact on generalizability than not having a correct ethnic representation. While most admitted that exclusion of ethnic minority patients would result in bias, many were confident the bias would not be significant. For example, Doctor 2 explained “We exclude those with reduced kidney function and liver function and those who’ve had jaundice and… No study reflects reality exactly; the study sample is usually healthier and has less morbidity…”
Another frequently mentioned aspect was the benefit of a very homogenous study sample. It was believed that a preferable option to having a diverse study sample would be to have many homogenous subgroups which can produce cleaner results and pinpoint the effect of new drugs. As one patient noted, “No, it’s actually good to have a homogenous patient group […] it’s easier to draw results, but the problem is how you’re supposed to generalize the result” (Subject 8).
There were few strategies identified by subjects, as these issues have not been perceived as problems and consequently no strategies have been formulated. A common conception was that exclusion of patients with a foreign background is very uncommon in Sweden: “I don’t know exactly how many patients aren’t included in studies because they don’t speak the language. I can imagine it’s a very small proportion” (Doctor 6). When asked about strategies, many subjects denied having any at all which also was attributed to the rarity of the issue: “I can tell you that I have never worked with this in any way. It hasn’t really been a problem the first 30 years of my life here, but we do see more foreign patients now” (Doctor 3).
A reason for this could be that the problem does not lie in the hands of researchers and healthcare, but rather on a broader, societal level: “I think we should put our efforts into education in the Swedish language […] and generally elevate the educational level […] I mean there are loads of socio-economic factors that we could try and equalize rather than turning the healthcare system upside-down” (Doctor 8).
Translating patient information was perceived to be the most effective solution to ensure inclusion of non-Swedish speaking patients. However, it was only believed to be worthwhile if the minority population speaking the language was significant enough to warrant the measure: “If we were a little more proactive and really felt that we wanted to reach all patients, you could already in the [research proposal describe] which languages patient information should be available in for maximal participation” (Doctor 4).
It was suggested that if patients could be justifiably included in trials after being given the information orally only, it might make it possible to use an interpreter: “But if they can understand some amount of Swedish and we have an interpreter then we can turn a blind eye and let them sign that they have understood what they’re signing” (Doctor 3).
Difficult information could also be explained by the doctor or with the help of a relative more fluent in Swedish: “I’ve always tried to get them to summarize the study in large and make them understand the core concepts, because that’s where the real ethical issue lies. Like ‘why are we doing this study?’ and ‘what’s the difference between treatment A and treatment B?’ and ‘what risks are included?’” (Doctor 4).
In many countries, like the US, but in contrast to Sweden, the ethnic or cultural origin of the patients is recorded and subsequently summarised. As a consequence, the misrepresentation of ethnic minorities in clinical trials has been well documented (Chen et al., 2014; Eshera et al., 2015). As Sweden has laws that prohibit the collection of data connected to ethnicity, comparable data is lacking. However, two previous studies performed in Sweden with patients in clinical cancer trials indicate low representation of ethnic minorities. A study of Bergenmar et al. (2011) asked which language was spoken at home instead of asking about ethnic origin. 95% answered that only Swedish was spoken at home. Another Swedish study indicated little ethnic variation as well (Godskesen et al., 2015).
This indicates selection bias also in Sweden, in line with the perceptions expressed in the interviews. In this study, all doctors had experience from situations where minority patients were excluded from clinical trials. Language barriers, lack of translated material and strict criteria for inclusion and exclusion set by ethical review boards were all considered as significant barriers for enrolling ethnic minority patients. There seemed to be a lack of strategies addressing the language issue, but the interview subjects had ideas about how exclusion might be prevented by using translated patient information and making the information process simpler and easier to understand. This corresponds to the literature (Durant et al., 2014; Hussain-Gambles et al., 2004). A primary reason given for the exclusion of linguistic minority patients is the lack of translated material or the added cost of translations or interpreters (Durant et al., 2014; Ford et al., 2008; George et al., 2014; Haley et al., 2017; Hughson et al., 2016; Kurt et al., 2016), leading to research becoming unavailable (Durant et al., 2014; Hussain-Gambles et al., 2004; Woodward-Kron et al., 2016).
Having adequate representation of the demographics of a study sample in clinical trials is essential to assess the safety and efficacy of new drugs. The subjects were not convinced that this kind of bias was relevant for their research studies as this problem was thought to be very uncommon. Though some doctors agreed that genetic differences might influence how well new drugs work, it was expressed that even if one would make an effort to include patients with other genetic traits, this would still drown in the final statistics. It was thus believed that the problem was too insignificant for any broader effort to counter it to be worthwhile. However, against this line of thought, it rather seems that we cannot know how much gain there is to be had from comparing outcomes, as we seem to lack the data needed to tell.
It is not only genetic differences that are of interest in this regard, but also e.g. issues of communication, such as how trust is built, side effects understood or verbalized, how symptoms develop, or issues of compliance. In other words, clinical trials do not exist in a vacuum; there are many other interesting issues to explore, and trials can themselves become the object of scientific studies. These can be significant to ethnic minorities and their health. The fairness of excluding those patients is, therefore, an issue. The issue can be regarded from a societal perspective, like what is seen in the US presently (Medical Press, 2018), where lawmakers are trying to help seriously ill patients by drafting a bill giving them a ‘right to try’ drugs being tested in clinical trials.
The physicians seemed to sometimes agree with an ethical imperative to promote fairness. But, as pointed out both in the introduction (Ford et al., 2008; Giuliano et al., 2000; Hussain-Gambles et al., 2004) and in the previous paragraph, there might exist individual preferences for participation as well as many general lessons to be learned about how to better care for ethnic minority groups and immigrants. Fairness is thus clearly at the heart of the issue and it deserves to be explored in more detail. On the other hand, the physicians’ reluctance to include ethnic minorities was motivated by a wish not to exploit a vulnerable group, so there is a balance to be struck here. Therefore, further discussion and research on this issue are required.
This explorative qualitative study has some limitations since it considers the experiences of a small sample of doctors (n=8). It does not provide a comprehensive overview of all physicians’ perspectives on recruiting ethnic minority participants to clinical trials and cannot say anything about the quantity and the generalizability of the problem. However, we underline that this was not the aim of this study; it was rather a qualitative exploration of how physicians encounter the issue of recruiting patients from ethnic minorities to clinical trials. As this issue, to our knowledge, is not previously analyzed in a Swedish context, it may serve as a stepping stone to further research on a subject rarely discussed. It should be noted that most of the physicians interviewed have long work experience, are from other hospitals in Sweden running clinical trials, and are very familiar with the issues discussed in the interviews. This strengthens the result.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The Authors declare that there is no conflict of interest.
Table 1. Participant Demographics
| Physicians (n=8) | |
| Gender | |
| Female | 4 |
| Male | 4 |
| Age (years) | |
| < 29-39 years | 3 |
| 40-49 years | 2 |
| 50-> 60 years | 3 |
| Working years in oncology | |
| Range | 3 |
| yrs | 1-30 |
| Mean/Median | 13/9 yrs |
| Practice setting | |
| Junior resident (still in training) | 1 |
| Specialist | 7 |
| Phase I | 4 |
| Phase II | 7 |
| Phase III | 7 |
Table 2: Interview Guide Used As a Basis for the Interviews
| Interview Questions |
| Which patients usually participate in clinical trials?
|
| How are you dealing with the recruitment of patients with a non-Swedish background? What strategies do you have?
|
| Do you think there is a problem with homogenous patient samples?
|
| How do you feel about including patients with language barriers and having trouble understanding the information about the study?
|
Bergenmar, M., Johansson, H., & Wilking, N. (2011). Levels of knowledge and perceived understanding among participants in cancer clinical trials – factors related to the informed consent procedure. Clinical Trials (London, England), 8(1), 77-84. doi:10.1177/1740774510384516
Britton, A., McKee, M., Black, N., McPherson, K., Sanderson, C., & Bain, C. (1999). Threats to applicability of randomised trials: exclusions and selective participation. Journal of Health Services Research & Policy, 4(2), 112-121. doi:10.1177/135581969900400210
Burnard, P., Gill, P., Stewart, K., Treasure, E., & Chadwick, B. (2008). Analysing and presenting qualitative data. British Dental Journal, 204(8), 429-432. doi:10.1038/sj.bdj.2008.292
Chen, M. L. (2006). Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet, 45(10), 957-964. doi:10.2165/00003088-200645100-00001
Chen, M. S., Lara, P. N., Dang, J. H., Paterniti, D. A., & Kelly, K. (2014). Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer, 120 Suppl 7, 1091-1096. doi:10.1002/cncr.28575
Durant, R. W., Wenzel, J. A., Scarinci, I. C., Paterniti, D. A., Fouad, M. N., Hurd, T. C., & Martin, M. Y. (2014). Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer, 120 Suppl 7, 1097-1105. doi:10.1002/cncr.28574
Eshera, N., Itana, H., Zhang, L., Soon, G., & Fadiran, E. O. (2015). Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA From 2010 to 2012. American Journal of Therapeutics, 22(6), 435-455. doi:10.1097/MJT.0000000000000177
Ford, J. G., Howerton, M. W., Lai, G. Y., Gary, T. L., Bolen, S., Gibbons, M. C., . . . Bass, E. B. (2008). Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer, 112(2), 228-242. doi:10.1002/cncr.23157
Ford, M. E., Siminoff, L. A., Pickelsimer, E., Mainous, A. G., Smith, D. W., Diaz, V. A., . . . Tilley, B. C. (2013). Unequal burden of disease, unequal participation in clinical trials: solutions from African American and Latino community members. Health and Social Work, 38(1), 29-38.
George, S., Duran, N., & Norris, K. (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16-31. doi:10.2105/AJPH.2013.301706
Giuliano, A. R., Mokuau, N., Hughes, C., Tortolero-Luna, G., Risendal, B., Ho, R. C. S., . . . McCaskill-Stevens, W. J. (2000). Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Annals of Epidemiology, 10(8 Suppl), S22-34.
Godskesen, T., Hansson, M. G., Nygren, P., Nordin, K., & Kihlbom, U. (2015). Hope for a cure and altruism are the main motives behind participation in phase 3 clinical cancer trials. Eur J Cancer Care (Engl), 24(1), 133-141. doi:10.1111/ecc.12184.
Haley, S. J., Southwick, L. E., Parikh, N. S., Rivera, J., Farrar-Edwards, D., & Boden-Albala, B. (2017). Barriers and Strategies for Recruitment of Racial and Ethnic Minorities: Perspectives from Neurological Clinical Research Coordinators. J Racial Ethn Health Disparities. doi:10.1007/s40615-016-0332-y.
Hansagi, H., & Allebeck, P. (1998). Enkät och intervju inom hälso- och sjukvård. Handbok för forskning och utvecklingsarbete. Stockholm: Studentlitteratur.
Hughson, J. A., Woodward-Kron, R., Parker, A., Hajek, J., Bresin, A., Knoch, U., . . . Story, D. (2016). A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials, 17(1), 263. doi:10.1186/s13063-016-1384-3.
Hussain-Gambles, M., Atkin, K., & Leese, B. (2004). Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community, 12(5), 382-388. doi:10.1111/j.1365-2524.2004.00507.x.
Kurt, A., Semler, L., Meyers, M., Porter, B. G., Jacoby, J. L., & Stello, B. (2016). Research Professionals’ Perspectives, Barriers, and Recommendations Regarding Minority Participation in Clinical Trials. J Racial Ethn Health Disparities. doi:10.1007/s40615-016-0322-0.
Medical Press. (2018). ‘Right to try’ seeks traction in US (Update). Retrieved from https://medicalxpress.com/news/2018-03-bill-advances.html.
Murthy, V. H., Krumholz, H. M., & Gross, C. P. (2004). Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA, 291(22), 2720-2726. doi:10.1001/jama.291.22.2720
Phan, V. H., Moore, M. M., McLachlan, A. J., Piquette-Miller, M., Xu, H., & Clarke, S. J. (2009). Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opinion on Drug Metabolism & Toxicology, 5(3), 243-257. doi:10.1517/17425250902800153.
SFS 2008:355 Patientdatalagen/Patient Data Act. Sweden.
SFS 2003:460. Lag om etikprövning av forskning som avser mäniskor/The Act concerning the Ethical Review of Research Involving Humans.
Woodward-Kron, R., Hughson, J. A., Parker, A., Bresin, A., Hajek, J., Knoch, U., . . . Story, D. (2016). Culturally and Linguistically Diverse Populations in Medical Research: Perceptions and Experiences of Older Italians, Their Families, Ethics Administrators and Researchers. J Public Health Res, 5(1), 667. doi:10.4081/jphr.2016.667.
World Medical Association [WMA]. (2013). Declaration of Helsinki. Retrieved from https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
Dr. Robert Spörndly is with the Centre for Research Ethics, Bioethics, Department of Public Health and Caring Sciences, at Uppsala University, in Uppsala, Sweden.
Dr. Stefan Eriksson is with the Centre for Research Ethics, Bioethics, Department of Public Health and Caring Sciences, at Uppsala University, in Uppsala, Sweden
Dr. Tove E. Godskesen is with the Centre for Research Ethics, Bioethics, Department of Public Health and Caring Sciences, at Uppsala University, in Uppsala, Sweden, and Ersta Sköndal Bräcke University College, Stockholm, Sweden
BCPHR.org was designed by ComputerAlly.com.
Visit BCPHR‘s publisher, the Boston Congress of Public Health (BCPH).
Email [email protected] for more information.
Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of BCPHR.![]()
© 2025-2026 Boston Congress of Public Health (BCPHR): An Academic, Peer-Reviewed Journal
All Boston Congress of Public Health (BCPH) branding and content, including logos, program and award names, and materials, are the property of BCPH and trademarked as such. BCPHR articles are published under Open Access license CC BY. All BCPHR branding falls under BCPH.
Use of BCPH content requires explicit, written permission.