Hughes C, Boeding T, Desai S, Westbrook K, Christian C, Patel A, Hoang T, Magadia R. Analysis of transmission rates in asymptomatic COVID-19 patients in a community hospital setting. HPHR. 2021; 29
DOI:10.54111/0001/cc13
Since January 20, 2020, there have been 4,405,932 cases of COVID-19 in the United States as of July 30, 2020 (1). The virus has been shown to spread via person-to-person transmission (2). There have been several reports of individual that have tested positive for SARS-CoV-2, but are otherwise asymptomatic (3, 4, 5, 6). Infectivity of asymptomatic carriers is imperative to the understanding of the disease process and has the potential to be a major public health challenge.
This study was conducted at the Regional Medical Center, a community hospital in Anniston, AL. We examined the outcomes of healthcare workers that were in contact with two patients that were asymptomatic during their stay, but were subsequently found to be positive upon discharge. The results of this study provide a better understanding of the risks of SARS-CoV-2 spread in asymptomatic patients with a focus on transmission to healthcare workers.
The RMC Human Resources Department released a list of both healthcare and non-healthcare employees who had been exposed to the two COVID-positive patients. Each individual was contacted via phone call or text to discuss the purpose of the study and to obtain the individual’s verbal consent. Out of 172 contacts, sixty-eight individuals agreed to be a part of the study. A 23-question survey was developed and emailed to those 68 subjects. The questions included COVID-related symptoms (including those in the household), medical history, current medications, and prior exposure to SARS-CoV 2. A follow-up survey was sent out via email three days after the initial survey was completed. The follow-up survey included symptoms that may have developed since submitting the initial survey, symptoms in household contacts, and use of PPE. Questions that were used in the initial survey and the follow-up survey can be found in Appendix A. Out of 68 subjects, 57 responded (response rate 83.8%). Answers for both surveys were identifiable by initials and the last 3 phone number digits for the purpose of continuity of follow-up.
The study was approved by IRB through the Alabama College of Osteopathic Medicine. The IRB number is HS200717EX. Official IRB letter of approval can be found in Appendix B.
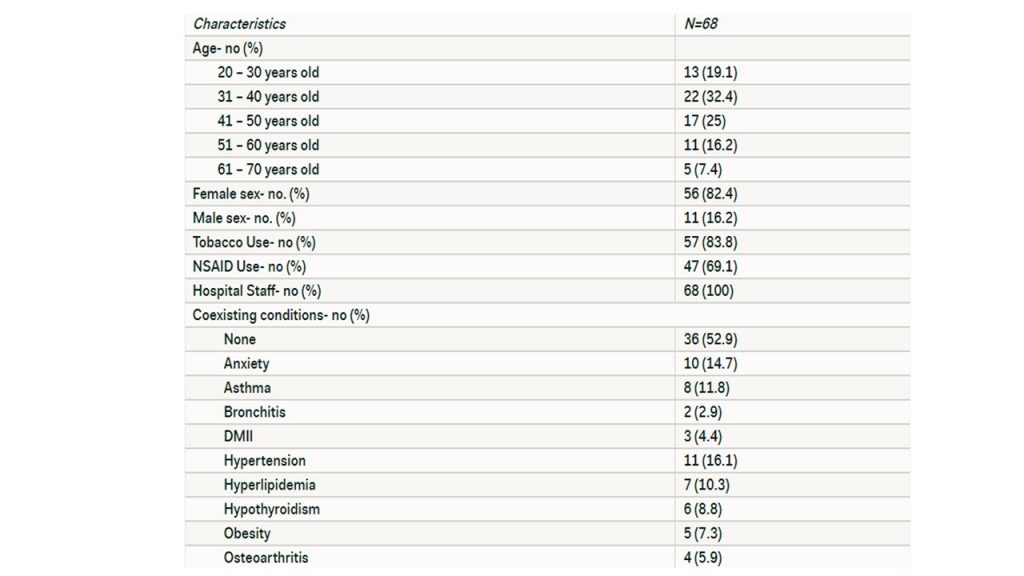
The demographics of the sixty-eight participants are listed in Table 1. The majority of participants were in the 41 to 50 year old age group. A majority of participants at 82.4% were of the female sex. Roughly 56 individuals or 83.8% have used or are currently using a tobacco product.
Thirty-four of the individuals who participated in the study stated that they have been in contact with other known COVID-19 patients while working at the hospital. Hospital protocol for known COVID-19 patients is respiratory droplet precautions with N95 and proper sanitization between each patient encounters.
Each of the individuals who participated in the survey had at least one encounter with the patient during their hospital stay. The number of exposures ranged from 1 to 30, with a median of 3 exposures, and an average of 4.5 encounters with the patient(s). The median time spent with the patient ranged from 1 min to 20 hours, with a median of 12 min, an average of 1.5 hours. The areas where the individuals came into contact include the hospital floor, emergency department, and procedural room. The roles of the individuals included but are not limited to nursing staff, physical therapy, dietician, phlebotomist, and respiratory therapy.
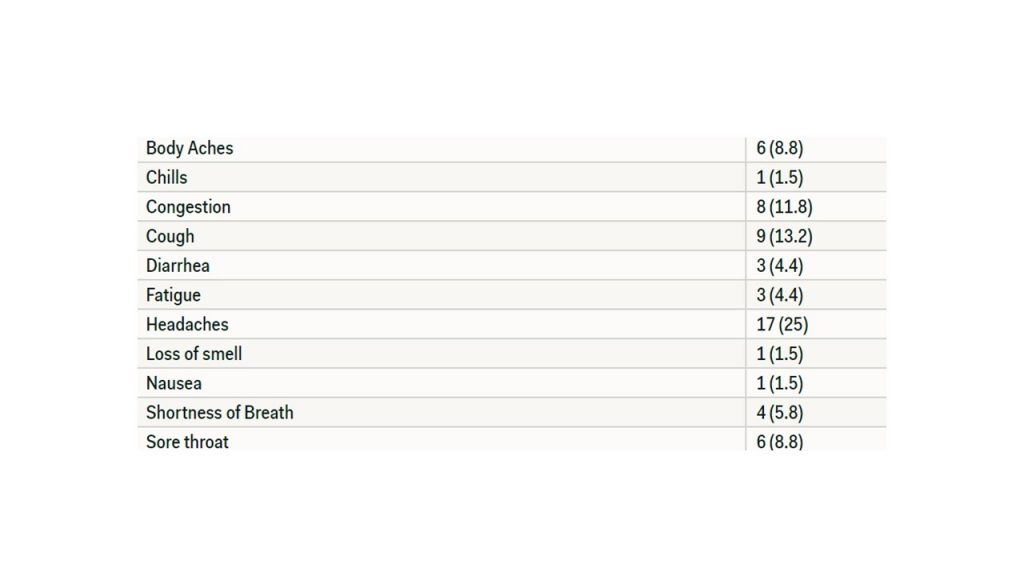
Of the 68 individuals who participated in the first survey, 44 of those individuals stated that they have not experienced any symptoms. Twenty-four individuals stated that they have experienced symptoms, which are listed in Table 2.
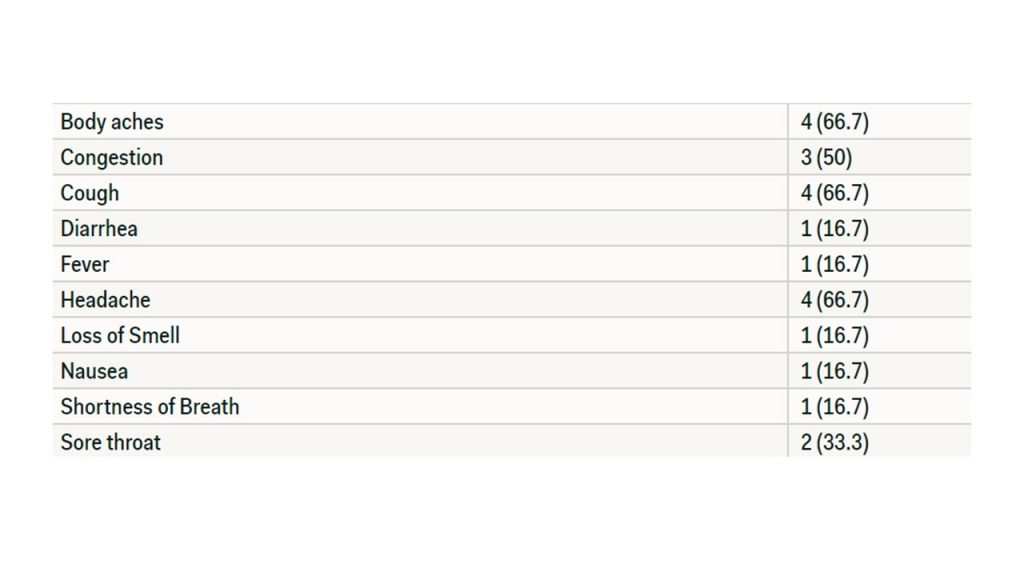
Of the 68 participants, six individuals stated that household members had begun showing symptoms. These individuals are considered the secondary contacts to the asymptomatic patients. These symptoms are listed in Table 3.
Of the 24 patients that reportedly developed symptoms in the first survey, the average amount of time spent with the patient(s) was 1.1 hours, the range of time was 0.083 to 12 hours, and the median of 0.1 hours. There were two values <0.1 hours in the data set.
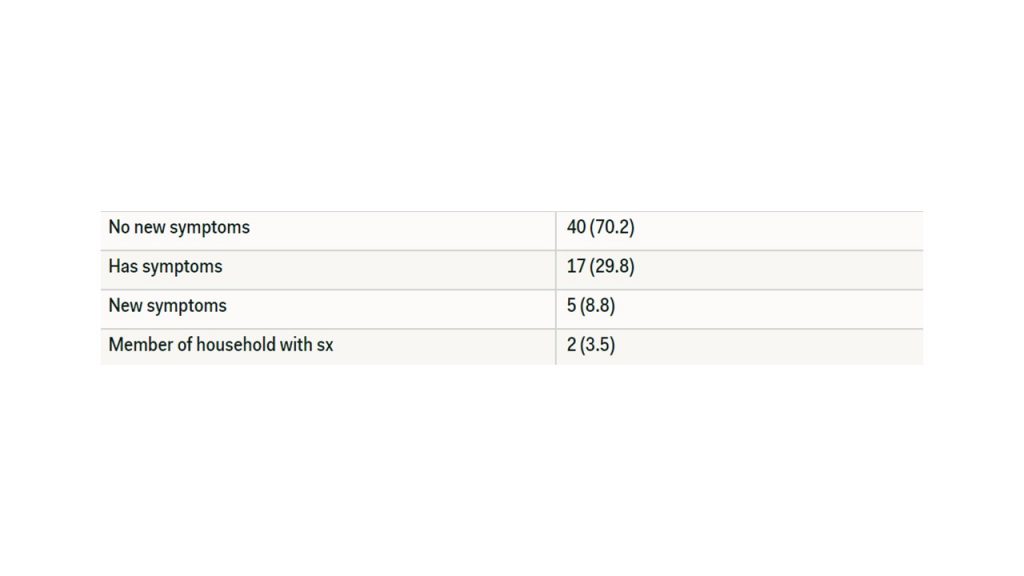
The follow up survey that was sent out 3 days after the administration of the first survey had 57 participants. Of these participants, three of the participants had developed new symptoms in a three-day period. These symptoms are as follows: body aches, headaches, diarrhea, bruising, blistering, and discoloration of the toes. In the second survey, two of the 57 individuals that has shown no symptoms stated that someone in their household had developed symptoms since the initial survey 3 days prior. These symptoms include body aches and a fever (temperature > 100.4 F). The results are summarized in Table 4.
The 33 individuals who later developed symptoms, including primary and secondary contacts, suggests a relatively low transmission rate of the virus, however, there are many variables that could have contributed to these low transmission rates. The first reason that the transmission rate was only 35% in the primary contacts (assuming that symptoms positively correlate with a + serology for SARS-CoV-2) is most likely due to the fact that population involved in this study were required by their employer to wear personal protective equipment such as N95 or surgical masks while in contact with patients. Various studies have confirmed that cloth masks reduce the rate of transmission by 87% (7) and N95 masks reduce the rate of transmission by 95% (8). Secondly, there appears to be a trend while looking at individual exposure times and the development of symptoms. The CDC states that anyone who is within 6 feet of an individual who has tested positive for SAR-CoV 2 for greater than 15 minutes is considered to be at risk of infection (9).
The study limitations don’t allow for definitive conclusions to be made. The initial participation rate of the study was 35.8%. The rate of response for the follow-up survey in the study was 83.8% from the participants in the first survey and 30% of the total exposure contacts. The time between the individual’s exposure to the asymptomatic patient to the time of the survey was on average about 15 to 30 days. This greatly limits the validity of the results and the ability to definitely say that there were not more individuals that were symptomatic during this time frame. Lastly, due to the wide array of clinical symptoms and lack of clinical testing available, it is difficult to say if the participant or their household contacts had truly developed COVID-19. While there are some pathognomic symptoms, it is difficult to derive a clinical diagnosis from these without confirmatory testing. This could have led to both under and over-reporting of the disease.
Given the results of this study, it continues to support the aspect of limiting the spread of COVID-19 through the use of protective equipment. This principle can be applied to both the healthcare and general public. Hopefully this study will help us approach a further clinical understanding of SARS-CoV 2 and how to protect those who become infected.
1.Cases in the US. (2020, June 26). Retrieved July 30, 2020, from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
2. Ghinai, I., McPherson, T., Hunter, J., Kirking, H., Christiansen, D., Joshi, K., . . . Illinois COVID-19 Investigation Team. (2020, April 4). First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Retrieved July 06, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7158585/
3. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic – Volume 26, Number 7-July 2020 – Emerging Infectious Diseases journal – CDC. (n.d.). Retrieved July 06, 2020, from https://wwwnc.cdc.gov/eid/article/26/7/20-1595_article
4. Bai, Y., Yao, L., Wei, T., Tian, F., Jin, D., Chen, L., & Wang, M. (2020, February 21). Presumed Asymptomatic Carrier Transmission of COVID-19. Retrieved July 06, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7042844/
5. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic – Volume 26, Number 7-July 2020 – Emerging Infectious Diseases journal – CDC. (n.d.). Retrieved July 06, 2020, from https://wwwnc.cdc.gov/eid/article/26/7/20-1595_article
6. Tan, J., Liu, S., Zhuang, L., Chen, L., Dong, M., Zhang, J., & Xin, Y. (2020, May). Transmission and clinical characteristics of asymptomatic patients with SARS-CoV-2 infection. Retrieved July 06, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7291769/
7. Rodriguez-Palacios, A., Cominelli, F., Basson, A., Pizarro, T., & Ilic, S. (2020, May 27). Textile Masks and Surface Covers-A Spray Simulation Method and a “Universal Droplet Reduction Model” Against Respiratory Pandemics. Retrieved July 31, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7267001/
8. Kilpatrick, R., & Meissner, H. (2020, July 29). How to reduce risk of COVID-19 transmission in outpatient settings. Retrieved July 31, 2020, from https://www.aappublications.org/news/2020/04/08/idsnapshot040820
9. Public Health Guidance for Community-Related Exposure. (n.d.). Retrieved July 31, 2020, from https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.htm
Questions used in initial COVID-19 Survey that was administered on day 0:
1. What is your identifier? (Please enter first and last initial with last 3 digits of your phone number)
2. What is your age?
3. What is the gender you were given at birth?
4. What is your race?
5. What is your ethnicity
6. Do you use or smoke tobacco?
7. Have you been diagnosed with any medical conditions?
8. What medical conditions have you been diagnosed with?
9. What medications are you currently taking for these conditions? (Please include prescription medications, over the counter medications, and any herbal or vitamin supplements that you are currently taking)
10. Do you use acetaminophen, aspiring, or any other over the counter anti-inflammatory medications?
11. Please check all symptoms you have experienced since coming into contact with the patient that has since tested positive for COVID-19?
12. Has there been anyone in your household showing any of the symptoms mentioned?
13. What are the symptoms that the household member been showing?
14. What was the date of your first encounter with this patient?
15. What was the date of the last encounter with this patient?
16. If you had contact with both patients, please list the first and last date of the second patient that was encountered below. (please indicate gender of patient in the response)
17. How many encounters did you have with this patient? (Encounters are considered periods of being within 6 feet of the patient. Leaving the room or caring for other patients then returning to the patient should be claimed as a separate encounter)
18. Can you estimate the total amount of time that was spent with the patient? (Please specify unit of time in answer)
19. Where did you encounter the patient?
20. What was your role in the care of the patient?
21. Have you had any other possible recent exposures to COVID-19 patients?
Questions used in the survey that was administered 3 days after the initial survey:
1. Please enter your patient identifier
2. Have you developed any new symptoms since the prior survey?
3. Please select any symptoms you have begun experiencing in the last 3 days.
4. Has anyone in your household begun to experience symptoms in the last 3 days?
5. Please select any symptoms people in your household have begun experiencing in the last 3 days.
6. Have you taken care of any COVID (+) patients during the last 3 days?
7. Have you used appropriate PPE when in close contact with COVID (+) patients?
8. Have you have any possible recent exposures to COVID (+) patients outside of the hospital? Please elaborate.
BCPHR.org was designed by ComputerAlly.com.
Visit BCPHR‘s publisher, the Boston Congress of Public Health (BCPH).
Email [email protected] for more information.
Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of BCPHR.![]()
© 2025-2026 Boston Congress of Public Health (BCPHR): An Academic, Peer-Reviewed Journal
All Boston Congress of Public Health (BCPH) branding and content, including logos, program and award names, and materials, are the property of BCPH and trademarked as such. BCPHR articles are published under Open Access license CC BY. All BCPHR branding falls under BCPH.
Use of BCPH content requires explicit, written permission.